Parvoruvax®


BROAD ERYSIPELAS PROTECTION
- Against all most common swine Erysipelas strains, 1a, 1b and 2
- Erysipelas may appear in:
- acute form: septicaemia, hyperthermia, anorexia, erythema and death
- sub acute /chronic forms: arthritis, endocarditis and orchitis
- indirect reproductive failure: return to heat, abortions, embryonic death, infertility and decreased seminal quality
PARVOVIRUS PROTECTION
- Against all strains of PPV causing reproductive problems to date
- Porcine parvovirus (PPV) is the major cause of reproductive failure in swine = SMEDI (stillbirth, mummification, embryonic death and infertility)
Piglets born viremic during infection
A proper diagnosis of Parvo and Erysipelas is crucial
Diagnosis of Parvovirus
|
Diagnosis of Erysipelas
|
VACCINATION IS THE ONLY WAY TO CONTROL THE DISEASES

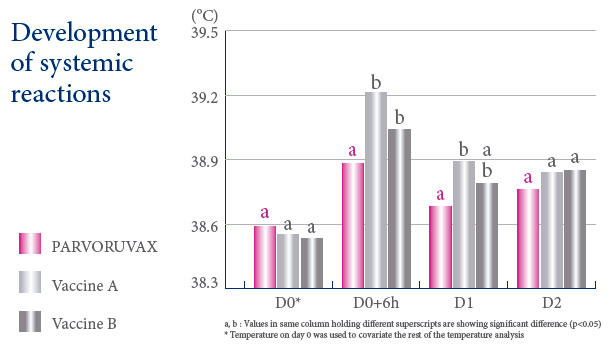
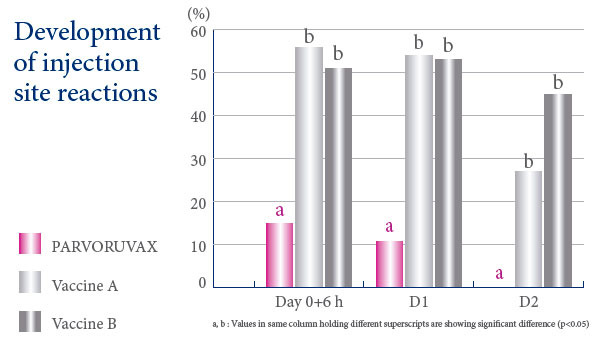
 A VACCINE WITH AN EXCELLENT SAFETY PROFILE
A VACCINE WITH AN EXCELLENT SAFETY PROFILE
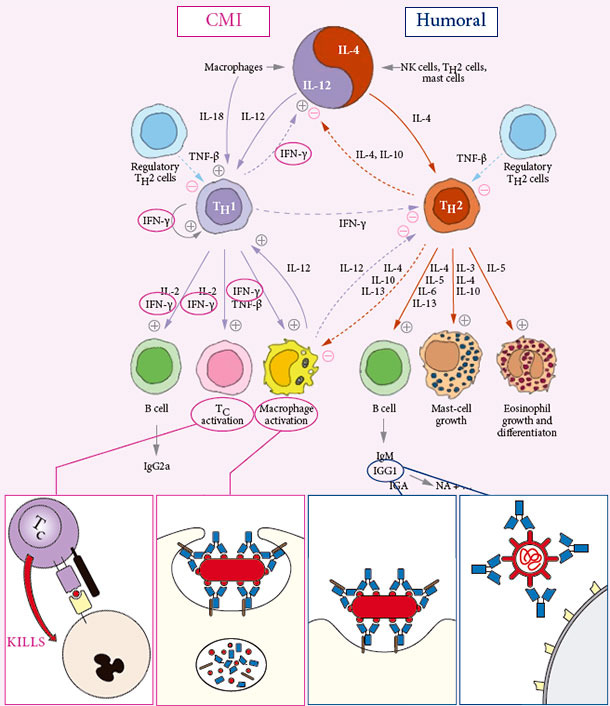
INDUCES BOTH CELL MEDIATED AND HUMORAL IMMUNITY
|
Specific protective Cell Mediated Immunity CMI is… CLEANING OUT INFECTION
|
Specific protective humoral immune response Humoral immunity is… PROTECTING AGAINST EFFECT OF INFECTION
|
The critical measures for DUAL PARVO AND ERY VACCINE PROTECTION are NA, Anti-SpaA & IFNγ-SC
Zeeuw et al., J Gen Virol 88 (2007) 420-27; Joswik et al., J Gen Virol 90 (2009) 2437-41; A.L. Ingebritson, Thesis ISU 2009; Crussard et al., IPVS/ESPHM 2016, 259
No correlation exists to general IgG ELISA´s or HI tests

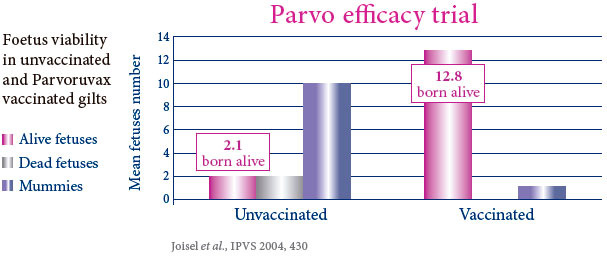
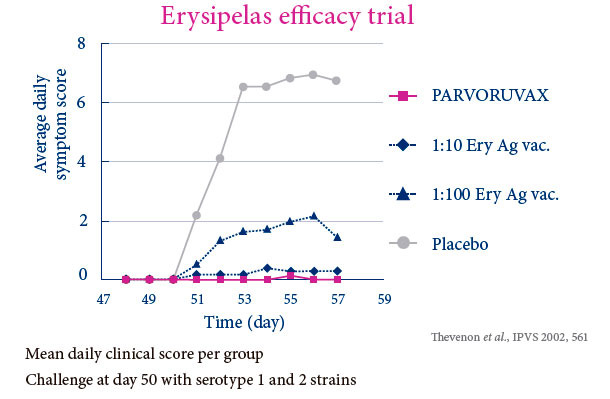
 PROVIDES POWERFUL AND BROAD DUAL PROTECTION AGAINST PARVOVIRUS AND ERYSIPELAS
PROVIDES POWERFUL AND BROAD DUAL PROTECTION AGAINST PARVOVIRUS AND ERYSIPELAS
IFNγ-SC/CMI
– a significant and persistent cell mediated immune response against PPV and Erysipelas
(Juillard et al., IPVS 2006, 162. Piras et al., IPVS 2006,160)
Neutralizing Antibodies
– a powerful and long lasting protective humoral immune response against PPV
(Brun et al., IPVS 2006,161)
Anti-SpaA Antibodies
– a very quick and strong protective humoral immune response against Erysipelas
• Even following first primo vaccination
• Specific Anti-SpaA antibodies as well as neutralizing antibodies are linked directly to protection
• Poor correlation to general Ig tests like HI & ELISA
• Commercial ELISA’s are designed on various antigens to discover previous field infection.
(Crussard et al., IPVS/ESPHM 2016, 259)
Parvoruvax® Suspension for Injection contains Inactivated Porcine Parvovirus (K-22 strain) and Erysipelothrix rhusiopathiae (lysed bacterial cells), serotype 2. Also contains aluminium hydroxide and thiomersal. The product is indicated for active immunisation of breeding pigs (sows, gilts and boars) against porcine parvovirosis, to reduce the number of stillbirths and mummified piglets, and against erysipelas to reduce or prevent clinical symptoms. Vaccination can occasionally cause reactions of hypersensitivity in some animals, particularly in those animals sensitised by the erysipelas infection. In such case, appropriate treatment such as adrenaline should be provided. Rarely, vaccination can induce a small local reaction (<1.5 cm) at the site of injection without any effect on the health or productivity of the animal. The vaccination can cause a slight rise in body temperature (<0.2°C) that returns to normal values from 1 to 2 days after vaccination without any consequence to the health or productivity of the animal. Primary vaccination against porcine parvovirosis should not be carried out in the presence of maternally derived antibodies. Only healthy animals should be vaccinated. The vaccine is safe for use during pregnancy and lactation. However, avoid vaccination during the 3 weeks following service mating. Withdrawal periods: Zero days. Pharmaceutical precautions: Use immediately after opening. Store between 2°C and 8°C, protected from light. For more details, see the SPC applicable in your country. This page contains information on a veterinary biological product sold in several different countries and areas where it may be subject to different regulatory approvals. Ceva gives no guarantee that the details presented are correct with respect to all locations. In addition, the safety and efficacy data and the withholding periods may be different depending on local regulations. Please consult your veterinarian for further information.
 Ceva Santé Animale
Ceva Santé Animale
10, av. de La Ballastière
33500 Libourne