Hyogen with Imuvant

M.hyo: a real economical problem for pig farmers
Mycoplasma hyopneumoniae, associated with enzootic pneumonia, plays a major role in the porcine respiratory disease complex (PRDC) and causes huge economic losses. A 17% decrease in daily weight gain and a 14% decrease in feed efficiency in herds with enzootic pneumonia has been reported1.
M. hyopneumoniae is unable to penetrate and live within host cells but does colonize the mucosal surface of the ciliated epithelium of the trachea and bronchi of pigs. In addition to the detrimental effect on the cilia, M. hyo infection attracts lymphocytes and macrophages into the lungs resulting in pneumonia. Protection against the infection requires complex activation of the host immune system.
Hyogen®: protect your pigs against Mycoplasma hyopneumoniae
After a single injection, Hyogen® provides effective protection against M. hyo due to the potent stimulation of the immune system by the antigen originated from Ceva M. hyo strain BA 2940-99 along with the adjuvant, Imuvant™.
Ceva proprietary strain BA 2940-99: To determine the best option, for a new vaccine, Ceva has decided not to use the old J strain but to make a survey to find a circulating virulent strain reflecting current field situation. The lab has also worked on proteomic and antigenic characterization of the strain to induce high level of immunity.
Imuvant™ is a unique Ceva adjuvant composed of a non-toxic lipopolysaccharide (LPS) from J5 E. coli together with a mineral oil in water emulsion that forms a complex and highly efficient immunostimulant2,3. This stimulation is due to the oil in water formula acting as a direct delivery system, which promotes the uptake of antigen by Antigen Presenting Cells (APC). At the same time, the LPS component of Imuvant™ stimulates the innate immune system while simultaneously enhancing the speci fic response to mycoplasma antigen via B and T lymphocyte activation pathways4.
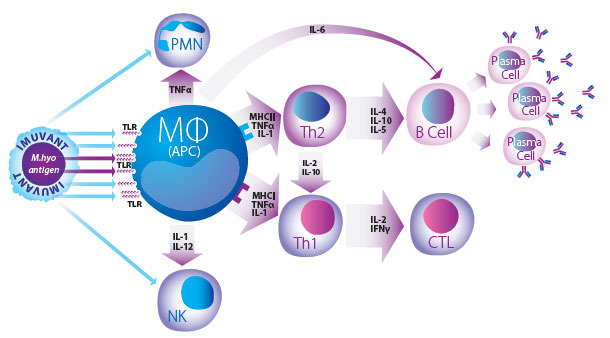
Innate, Humoral and Cell Mediated Immunity
Cellular Immunity Hyogen® vaccinated pigs had significantly higher mean M. hyo specific, IFN γ producing, white blood cell counts as compared to the controls5.
Mean Number of Antigen Specific White Blood Cells / 6 log 10 Peripheral Blood Mononuclear Cells
| Day 13 after vaccination |
Day 90 after vaccination |
Day 175 after vaccination |
|
| Hyogen® Vaccinated | 23.75 | 25.31 | 10.38 |
| Control | 0.56 | 1.64 | 2.0 |
Humoral imunity Hyogen® also elicits a significant early and long lasting antibody response after vaccination as demonstrated in both onset of immunity and duration of immunity studies6.
Mean Antibody Titer Post Vaccination (EU/ml)
| Day 0 | Day 15 after vaccination |
Day 0 | Day 175 after vaccination |
||
| Hyogen® Vaccinated |
Negative | 2.4 | Negative | 6.1 | |
| Control | Negative | Negative | Negative | Negative |
IMUVANT™ INCREASES THE DEFENSE OF PIGS VACCINATED WITH HYOGEN® AGAINST MYCOPLASMA HYOPNEUMONIAE
|
In a clinical trial seronegative 3 week old pigs were either vaccinated with Hyogen® or served as non-vaccinated controls. All animals were challenged with M. hyopneumoniae day 17 post vaccination, and euthanized and necropsied on day 46. Hyogen® vaccinated animals had 19.9 times lower lung scores than the nonvaccinated controls (p = 0.0028)7. |
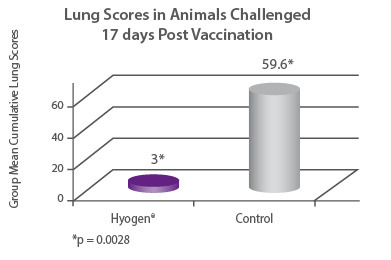 |
|
Seronegative pigs were vaccinated with Hyogen® at three weeks of age or served as non- vaccinated controls. Then 26 weeks after vaccination both groups were challenged with a virulent strain of M. hyopneumoniae and after 4 weeks were euthanized and necropsied. The Hyogen® vaccinated group had 14.32 times fewer lung (p = 0.0001)6. |
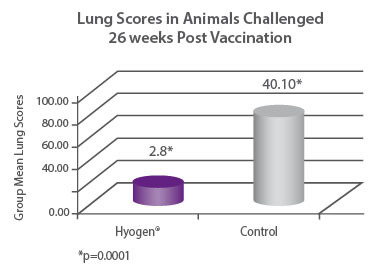 |
|
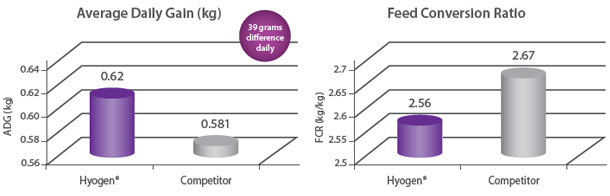
Protection of lungs In a field study involving 300 piglets on a farm where M. hyopneumoniae was present, pigs were vaccinated at three weeks of age with either Hyogen®, a competitor M. hyo bacterin or a placebo. Hyogen® vaccinated pigs had statistically significant lower lung scores at slaughter. Hyogen® is both efficacious in the face of M. hyo challenge in the field and provides significantly better protection than competitor vaccine8. |
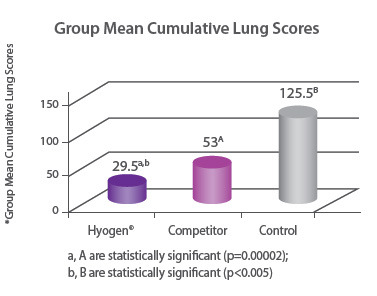 |
|
Protection of growth The correlation between the lung lesion scores and growth performance was demonstrated in the field trial where 350 piglets were vaccinated either with Hyogen® or a competitor vaccine at three weeks of age. At slaughter, Hyogen® vaccinated pigs had statistically significant lower lung scores and performed better than the competitor controls9. |
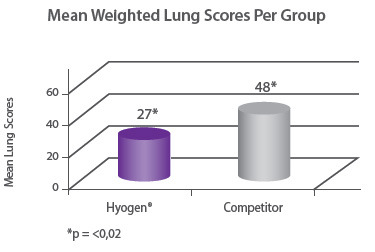 |
 |
|
Hyogen® is part of the Ceva Lung Program. The Ceva Lung Program provides an overview of the diseases associated with Actinobacillus pleuropeumoniae, Mycoplasma hyopneumoniae and Aujeszky’s disease virus. It offers the methodology and guidelines on how to correctly evaluate the presence, incidence, circulation patterns and impact of these infections using serological investigation and adapted lung scoring of slaughter pigs. It is used to determine the appropriate vaccination protocol and monitor the results of vaccination with Coglapix®,
Hyogen® and Auphyl® Plus. Overall, the program is supported by key opinion leaders and is an asset for building Ceva’s reputation as experts in respiratory health.
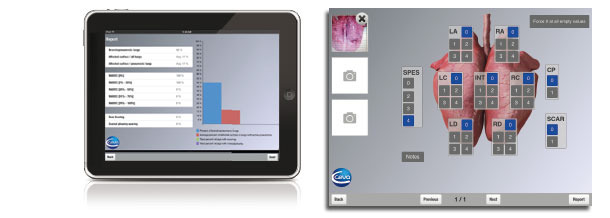
An iPad application to effectively score the lungs at the slaughterhouse.
• Single injection provides early and long lasting protection
• Broad stimulation of the immune system with ImuvantTM
• Trials demonstrate superior protection versus competitors8,9
• Expertise of the Ceva Lung Program
Superior Enzootic Pneumonia Protection
Posology and method of administration: Intramuscular route. Administer a single 2 ml dose from 21 days of age. Withdrawal time: Zero days. Special precautions for storage: The product must be stored between +2 and +8 °C protected from light. Do not freeze. Fore more details, see the SPC applicable in your country.
1. Straw 1989
2. Janeway, et al., Immuno Biology: The immune system in health and disease. 2005
3. Park, et al., Nature 453: 1191-1196, 2009
4. Lo. D-Y et al., J.Vet.Med.Sci. 71: 897-903, 2009
5. Herczeg et al., APVS 2011, «DOI study»
6. Ceva internal studies
7. Herczeg et al., APVS 2011, «OOI study»
8. Herczeg et al., IPVS 2012, p. 178
9. Herczeg et al., IPVS 2012, p. 204
 Ceva Santé Animale S.A.
Ceva Santé Animale S.A.
www.ceva.com
lungprogram@ceva.com
10, av. de La Ballastière - 33500 Libourne - France - Phone: 00 33 (0) 5 57 55 40 40